Einstein: "God does not play dice."
Einstein: "God is not malicious."
Bohr: "Einstein, stop telling God what to do."
"You are not thinking.
You are merely being logical."
-- Niels Bohr to Albert Einstein

One of the most sparkling and prolonged scientific jousting matches took place between Niels Bohr and Albert Einstein in the 20s and 30s. The latter, who could never accept the probabilistic nature of quantum mechanics, produced a series of gedanken experiments (thought experiments) designed to disprove the new theory. Bohr would then attempt to show where Einstein had gone wrong. In one of Bohr's successful attempts at this, he was especially pleased to note that Einstein had forgotten that according to his own theory of general relativity clocks run more slowly under the influence of a gravitational field.
In terms of scientific brilliance Niels Bohr is right at the top, perhaps second only to Einstein in the hit parade of 20th century scientists. It seems that every scientist who met Bohr came away with an impression of his deep intellect and his kind, gentle manner. (The phrase "large domed head" seems to occur frequently, too.)
Bohr lies at the end of a list of philosopher/scientists which charts the establishment of atomism in the scientific canon over the centuries: from the pre-Socratics Democritus and Leucippus;to Epicurus and Lucretius; to Dalton who was the first to create the concept of atoms in a modern scientific format; to Rutherford and Geiger who demonstrated the nuclear structure of the atom; to Bohr who was able to unite Rutherford's atom with the quantum concept of Planck.
Niels Bohr entered Copenhagen University in 1903, emerging in 1911 with his doctorate. (As if all his scientific gifts were not enough, Bohr was a shit-hot football player at university.) He then went on to study at Cambridge under J.J. Thompson then to Manchester under Rutherford. Rutherford was much more receptive to Bohr's new ideas and the two of them formed a very close personal and working relationship which resulted in Bohr producing his theory of the hydrogen atom in 1913 at the age of 27.
In 1911, Rutherford had postulated an atomic model which described the hydrogen atom as a small heavy nucleus surrounded by an electron in a fixed circular orbit around it. The only snag here was that this arrangement was completely forbidden by the laws of classical physics. According to Maxwell's equations, the electron, involved in circular motion hence accelerating, should be continuously emitting electromagnetic radiation. This energy could only come from the rotational motion, so the electron should spiral into the nucleus
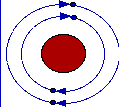
Bohr's masterstroke was to say that an electron was able to orbit the nucleus without emitting energy. Now, this mighty contribution to the advance of human knowledge might not have been so mighty had it not been accompanied by some more theory. Bohr said that many non-radiating orbits were allowed, each one having an integral factor of the basic unit of angular momentum, h/2*pi. When the atom absorbs radiation the electron is promoted from one fixed orbit to one of a higher energy (larger radius) and the energy of the photon is equal to the energy gap between the two orbits. Similarly, when an electron falls down from a higher energy orbit to a lower one it emits a photon of energy equal to the difference in energy between the two orbits. Thus the line spectrum of the hydrogen atom had been explained and the empirical formulae of Ritz, Rydberg and Balmer had received a theoretical explanation.
In 1916 Bohr returned to the University of Copenhagen as professor of physics where in 1920 they erected a special building to house the Niels Bohr Institute. Bohr's institute was supported financially by the Carlsberg brewing company, so it was ... yes ... probably the best institute of theoretical physics in the world ......
In the next decade this became the centre of the world for theoretical physics. Bohr's "Copenhagen school" produced the famous "Copenhagen interpretation" of quantum mechanics of which one of the main planks was Bohr's theory of "complementarity". For example, an electron can be regarded in two complementary ways - as a particle or a wave phenomenon with either being equally valid depending on the experimental circumstances.
One memorable quote from Bohr emerged after he had tried to explain quantum theory to a lecture room full of philosophers. He was amazed to find that all the philosophers sat there calmly and seemed to accept all the new ideas with nary a raise of the eyebrow. "Anyone who is not dizzy after his first acquaintance with the quantum of action has not understood a word", he said.